Which of the Following Species Is the Strongest Oxidizing Agent
NaI 3HOCl NaIO3 3HCl A. A since F2 has a the most positive potential and is on the left side.
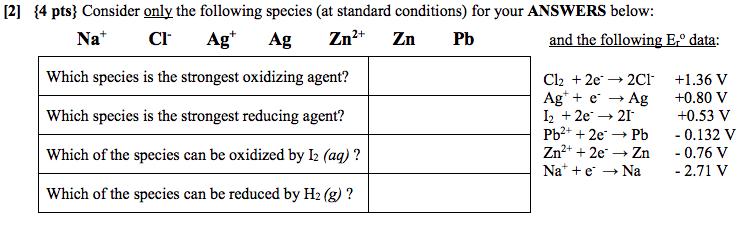
Solved 12 4 Pts Consider Only The Following Species At Chegg Com
HOCl is the oxidizing agent B.

. Examine the following half-reactions and select the strongest oxidizing agent among the species listed. Which of the following is the strongest oxidizing agent. NaI is the reducing agent D.
A Fe electrode in 10 M FeCl2 solution. Which species is the strongest oxidising agent. Refer to electrochemical seriesIf all species are in their standard states which of the following is the strongest oxidizing agent.
A Zns b Mgs c Al3aq d Mg2aq Chemistry. The strongest oxidizing agent among the species I n 3 E 1. The following two half-reactions take place in a galvanic cell.
At standard conditions what species are produced at each electrode. E 0691 V for Ag 2 Ss 2e Ags S 2 aq which is too negative for Ag 2 S to be spontaneously reduced by oxalic acid E 049 V for 2CO 2 g 2H aq 2e H 2 C 2 O 4 aq. 3 4 V A u 3 E 1.
Which species is the strongest oxidising agent. 8 6 V C r 3 E 0. A F2 BF-CLi DLi GIVEN THE TABLE ON THE OTHER SIDE OF THIS CARD.
Home Community Which of the following is the strongest oxidizing species. 4 V H g 2 E 0. I-aq is a stronger oxidizing agent than Nis and I2s is a stronger reducing agent than Ni2aq.
7 4 V is. If all species are in their standard states which of the following is the strongest oxidizing agent. Consider the following standard reduction potentials Ni2aq 2 e- Nis E -026 V I2s 2 e- 2 I-aq E 054 V Under standard conditions A.
Cr2aq 2e- CrsE -0913 V Fe2aq 2e- FesE -0447 V Sr2aq 2e- SrsE -289 V Co2aq 2e-. Consider the following reaction and select the false statement below. Which speciesSn 4 aq Cl aq Ag aq Cr 3 aq andor H 2 O 2 aqis the strongest oxidizing agent in aqueous solution.
MnO 4 4H 3e MnO 2 2H 2 O E 168 V I 2 E 2e 2I 054 V Zn2 2e Zn E -076 V A MnO 4 B I 2 C Zn2 D Zn E MnO 2 4. Consider an electrochemical cell constructed from the following half cells linked by a KCl salt bridge. A Na b Li c Ba2 d Mg2 The answer to the question is d but I dont know why.
Examine the following half-reactions and select the strongest oxidizing agent among the species listed. 1 Sn2 2 Sn4 3 Br2 4 Br-5 Li. A Na b Li c Ba2 d Mg2 The answer to the question is d but I dont know why.
A Sn electrode in 10 M Sn NO3 2 solution. Which of the following is the strongest oxidizing agent. Which of the following species is the strongest oxidizing agent.
Cr2aq 2e- Crs E -0913 V Fe2aq 2e- Fes E -0447 V Sr2aq 2e- Srs E -289 V Co2aq 2e- Cos E -028 V. Which of the following species is the strongest oxidizing agent. Which species is the strongest oxidising agent.
Very Important Questions Rain is falling at the speed of. For F 2 E o RP is minimum. The strongest oxidising agent among the following is_____.
Calculate Ecell for a silver-aluminum cell in which the cell reaction is. Cl is reduced C. 5 Which of the following is the strongest oxidizing agent.
Heres a typical table of standard reduction potentials. The strongest oxidizing agent in the list is F2 followed by H2O2 and so on down to the weakest oxidizing agent Li. Which substance is the best oxidizing agent among following.
Al s 3Ag aq Al3 aq 3Ag s 246 V. Answer - The strongest oxidising agent from the given option is O2 g with standard View the full answer. View solution Which one of the following is the strongest oxidising agent.
The more positive the potential the stronger or weaker the oxidizing agent. F 2 is the best oxidizing agent of the periodic table. The species at the top left have the greatest potential to be reduced so they are the strongest oxidizing agents.

Solved Which Of The Following Species Is The Strongest Chegg Com
What Is The Strongest And Weakest Oxidising And Reducing Agents Quora

Solved Which Of The Following Species Is The Strongest Chegg Com
0 Response to "Which of the Following Species Is the Strongest Oxidizing Agent"
Post a Comment